A new class of proximity-inducing bifunctional molecules called covalent glue mimics (CGMs) promises to revolutionize cancer immunotherapy by enabling stable interactions between immune cells and tumor cells.
These innovative molecules utilize a dual proximity labeling strategy to facilitate the covalent crosslinking of a tumor surface protein with an antibody, effectively enhancing immune recognition and targeting of cancerous cells.
The researchers, from an international team of biological and chemical scientists, have pioneered this approach to address a major challenge in cancer treatment: the formation of stable complexes between non-interacting proteins in the presence of competitive biological inhibitors.
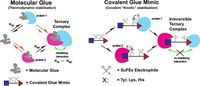
Molecular glues, while conceptually promising, have historically been difficult to design for scenarios involving proteins that do not interact favorably. This has especially been the case in scenarios where two cells must be brought into close proximity to activate an immune response against tumors.
The study outlines how CGMs resolve this issue by mimicking the stabilizing function of molecular glues. By strategically incorporating dual electrophiles that form irreversible bonds once the target proteins are engaged, CGMs create a stable environment resistant to perturbations from competitive ligands.
A prominent obstacle in cancer immunotherapy is ensuring robust cell-cell interactions, as the dynamics of protein interactions can often lead to dissociation instead of stable binding. This is especially relevant when considering the activity of immune effector cells against malignant cells.
In the face of conventional binding strategies that depend on the presence and concentration of the involved proteins in the tumor microenvironment, CGMs exhibit enhanced functional properties that facilitate better engagement and longer-lasting interactions.
In laboratory models, the CGMs demonstrated significant improvements in forming stable complexes in multiple tumor-immune recognition scenarios. For example, they effectively prevented the targeting immune cells from losing their grip on tumor cells, thus enhancing antitumor activity.
This advancement not only signifies a leap toward more effective cancer treatments but also showcases the broader implications of using materials that can circumvent the natural barriers created by biological inhibitors.
Study Challenges Traditional Drug Design
The innovation of CGMs challenges traditional drug design paradigms by demonstrating that stable interactions can be accomplished even in cases where the proteins within the ternary complex would typically interact unfavorably.
One standout finding from the study is that the covalent bond formed by CGMs effectively reinforces the association of proteins, thus promoting the functional synergy achieved in co-localized immune response dynamics.
Although the study identifies the potential of CGMs in clinical applications, the authors stress the limitations encountered in vivo regarding rapid clearance rates and potential off-target toxicity.
The implications of this research extend beyond oncology and suggest future uses in various therapeutic domains where enhancing the efficacy of bifunctional binding dynamics can lead to improvements in precision medicine.
Ultimately, this study represents a significant advancement in both drug development processes and our understanding of protein interactions within therapeutic contexts. As researchers continue to refine the design and versatility of CGMs, we can anticipate a new era in how bifunctional molecules can be leveraged against complex diseases like cancer.