The emergence of SARS-CoV-2 variants has posed significant challenges to global health, necessitating a thorough understanding of how the immune response can be optimized through vaccination. New research out of the University of Oxford reveals that the complement system—a part of the innate immune response—can dramatically enhance the SARS-CoV-2 antibody neutralization titres, with improvements observed of up to 83-fold against the Omicron variant BA.1.
As the pandemic progresses, concerns have arisen concerning the efficacy of vaccines against evolving variants. Traditional neutralization assays often neglect the complement system's contribution, leading researchers to assess its role more thoroughly. Previous studies had indicated that complement significantly boosts the neutralizing activity of antibodies across a spectrum of viruses, yet this relationship had not been fully explored in the context of SARS-CoV-2.
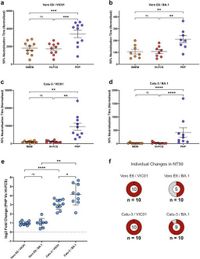
In the current study, researchers designed microneutralization assays using serum from vaccinated individuals. They introduced pooled human plasma (PHP) to replenish the complement system's activity, revealing that this enhancement varied remarkably among different individuals, viral strains, and cell lines.
Notably, findings indicated that the presence of complement proteins significantly increased neutralization titres against both wild-type and Omicron variants. For instance, they demonstrated that the neutralization of the BA.1 strain was entirely dependent on complement in some sera, especially when cross-reactivity was assessed.
Utilizing laboratory techniques, the scientists found that not only were the neutralization capacities improved, but these enhancements appeared to stem from the complement proteins’ ability to interfere with spike-ACE2 interactions, a critical step required for the virus’s entry into human cells. C3, one of the complement proteins, emerged as essential for achieving maximum efficacy in this neutralization process.
The methodology involved heating plasma, which inactivated complement activity, underscoring the importance of using non-heat-inactivated plasma in these assays to preserve its functionality. The results indicate that relying solely on conventional assays without the complement system could yield misleading assessments of vaccine effectiveness.
This enhancement of neutralization efficacy has vital implications for vaccine development and therapeutic strategies, particularly as SARS-CoV-2 continues to evolve. The study suggests that integrating the evaluation of complement activity into future research efforts may enhance our understanding of vaccine-induced immunity and guide the enhancement of existing vaccines to ensure continued protection against potent variants.
A cohort of vaccinated healthcare workers provided the serum analyzed, strengthening the study's relevance to real-world contexts. Future studies will likely explore the mechanistic insights further, particularly regarding how individual variations in antibody profiles may influence complement activation and overall immunity.
Overall, understanding the interplay between antibodies and the complement system may provide critical insights into improving vaccine design and therapeutic interventions for COVID-19, especially as the world continues to grapple with ongoing and emerging variants of concern. These findings highlight the necessity to recalibrate our approaches to vaccine efficacy assessments in light of the evolving understanding of humoral immunity.
This research not only reinforces the integral role of the complement system in antibody mediated neutralization but also raises questions about the adaptability of our current vaccination strategies. As the landscape of COVID-19 challenges shifts, the need for robust protective measures becomes increasingly clear, paving the way for future research endeavors aimed at bolstering global health against this viral threat.