The study of embryonic stem cells (ES cells) has revealed complex relationships between protein complexes responsible for maintaining chromosomal structure and function. A recent investigation has provided new insights into how two such complexes, cohesin and condensin, interact to ensure accurate chromosome segregation during cell division while also contributing to the maintenance of pluripotency in these cells.
Published on March 22, 2025, the research, conducted by scientists E.H. Choi and K.P. Kim, employed advanced techniques, including ChIP-seq and RNA sequencing, to unravel the roles of cohesin and condensin in ES cells. These exceptional cells possess the ability to self-renew and develop into various cell types, making them a key focus in regenerative medicine.
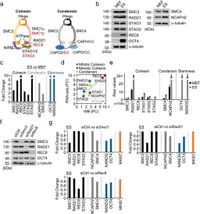
Cohesin is a ring-shaped protein complex that holds sister chromatids together until their separation in the anaphase of cell division. It consists of several subunits, including SMC3, RAD21, and REC8. Condensin, on the other hand, facilitates the compaction and proper segregation of chromosomes during mitosis. This study highlighted a crucial aspect of their relationship—how they share DNA binding sites and interact with the insulator protein CTCF, which is instrumental for maintaining the topological structure of chromatin.
One of the striking findings of this research is the unique presence of meiotic cohesin complexes in ES cells, specifically those containing the kleisin subunit REC8. These complexes were shown to play a significant role in the mitotic program of such cells. The authors noted, "Cohesin and condensin make important contributions to the functions of the chromosomal organization, and that meiotic cohesin may be specifically required for the mitotic program in ES cells." This highlights the dynamic nature of these complexes in facilitating cellular processes crucial for stem cell function.
The researchers also explored the effects of knocking down various cohesin components using siRNA. They discovered that silencing SMC3 led to an intriguing increase in the nuclear accumulation of SMC4, a core factor of the condensin complex. This suggests a compensatory mechanism where, under conditions of cohesin depletion, condensin may relocate to maintain chromosomal integrity.
In detail, when both RAD21 and REC8 were knocked down, the transcription levels of genes associated with pluripotency, such as NANOG and OCT4, were significantly decreased—over 20% for each. This emphasizes the critical role that these complexes play in regulating key genes necessary for maintaining the undifferentiated state of ES cells. As the authors reported, "The upregulated genes were associated with apoptotic processes, cell death, and inflammatory responses, whereas the top downregulated genes were enriched in translation, DNA repair, chromosome organization, and cell differentiation." This finding underscores the balance these complexes must achieve to preserve pluripotency while preparing cells for differentiation.
The overlap in DNA-binding sites between cohesin and condensin complexes was particularly remarkable. The research demonstrated that both shared genomic locations critical for maintaining the chromatin structure and each other's positions at these sites. Such an interaction can facilitate long-range genomic interactions, crucial for proper chromosomal behavior during the cell cycle.
To examine these interactions more closely, the study also involved co-immunoprecipitation assays, which revealed a strong interaction between SMC3 with both RAD21 and REC8. However, further analysis showed that disruption of RAD21 resulted in a notable increase—by 1.67-fold—in REC8 levels, signifying a complex regulatory system between these proteins that adapts to the cellular context.
These findings suggest substantial adaptive capacity within cohesin complexes in response to genetic or environmental stresses faced by embryonic stem cells. It sheds light on the intricate processes that sustain the pluripotent state, foundational for potential therapeutic applications in regenerative medicine. The adaptability observed not only enhances our understanding of chromosomal mechanics but also opens new avenues to explore the implications of cohesin and condensin interactions in various diseases linked to chromosomal instability.
In conclusion, the interplay between cohesin and condensin in maintaining chromosomal architecture is crucial for the integrity and functionality of embryonic stem cells. Understanding these relationships may not only advance our grasp of fundamental biological processes but also lead to innovative strategies to address cohesinopathies and other chromosomal disorders. Future research directions should focus on elucidating the precise mechanisms that govern the interactions between RAD21 and REC8 and investigating how these complexes can be manipulated for therapeutic benefit.