Recent research has uncovered vital insights into the role of a protein called centrobin in the maturation of centrioles, which are essential structures for cell division and cilia formation. Conducted by a team of researchers, the study meticulously traced centrobin's localization throughout the cell cycle, revealing its crucial functions and potential implications for human health.
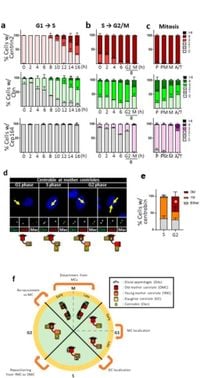
Centrioles serve as the main organizing center for microtubules in animal cells, allowing cells to divide properly and generate cilia—tiny hair-like structures on the cell surface that play important roles in cellular signaling and movement. This study findings demonstrate that centrobin plays an instrumental role in ensuring that daughter centrioles mature accurately into functional structures required for these processes.
As the cell cycle progresses, daughter centrioles begin to assemble during the S phase and, following the M phase, transform into young mother centrioles. This transition is key for the centrioles as they will ultimately need to form distal appendages (DAs) that enable the generation of cilia. Centrobin remarkably affixes itself to nascent daughter centrioles during the S phase and detaches from young mother centrioles just before the G2 phase, providing a temporal safeguard against premature maturation.
In exploring the mechanics behind this regulation, researchers employed various methodologies. Through a process known as CRISPR/Cas9 gene editing, they created centrobin knockout (KO) cell lines in human HeLa and mouse embryonic fibroblast (MEF) cells to observe the consequences of lacking this protein. These experiments revealed that without centrobin, daughter centrioles exhibited premature installation of DAs. This aberration resulted in the production of doublet cilia—two cilia generated from one basal body, which might impair normal cellular function.
Moreover, the study identified a crucial relationship between centrobin and Polo-like kinase 1 (Plk1). Findings suggest that direct phosphorylation of centrobin by Plk1 is essential for its attachment to centrioles during the cell cycle. This regulation emphasizes the coordination needed between biochemical signals and structural integrity in centriole maturation.
Centrobin KO mice further confirmed the pivotal role of the protein in vivo. While these mice were found to be viable, they exhibited male sterility and a notable reduction in the size of their testes. Histological examinations revealed a significant decrease in post-meiotic male germ cells, further demonstrating how critical centrobin is for reproductive health.
In terms of broader implications, failures in cilia formation are linked to various human diseases, known as ciliopathies. Thus, understanding the mechanisms behind centriole maturation and centrobin's role could provide insights into the treatment of such diseases. The data indicate that appropriate regulation of DA formation is not only necessary for successful cell division but also for maintaining normal cilia function.
The study ultimately presents a compelling case for centrobin as a key player in safeguarding timely centriole maturation within the cell cycle. As scientists delve deeper into the complexities of centriole behavior and regulation, the prospects for new therapeutic approaches to ciliopathies and fertility issues continue to grow.