In a groundbreaking study, researchers have discovered that the intratracheal administration of the fungus Candida albicans significantly worsens lung hemorrhage in mice with lupus-like symptoms. This research sheds light on the potent interactions between fungal presence in the lungs and autoimmune responses associated with systemic lupus erythematosus (SLE).
Systemic lupus erythematosus is an autoimmune disease affecting approximately 100 individuals per 100,000, with respiratory involvement reported in 50-70% of patients. Among these complications is diffuse alveolar hemorrhage (DAH), which affects a smaller percentage—between 0.5-9%—yet carries high mortality, especially with frequent recurrent episodes.
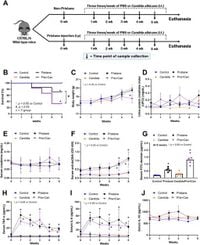
The study, published on March 21, 2025, explores the impact of Candida on the severity of lung-related symptoms in a pristane-induced mouse model. The researchers focused on how intratracheal administration of Candida affects the central symptoms associated with DAH and lung microbiota.
Recent findings indicated that the administration of Candida worsened mortality rates, body weight, and specific serum cytokines (TNF-α and IL-6) over five weeks. The lung hemorrhage score significantly increased for mice receiving both pristane and C. albicans compared to those treated with pristane alone.
"Candida administration worsened mortality, body weight, serum cytokines, and lung hemorrhage score at 5 weeks of the model,” wrote the authors of the article, highlighting the potential mechanistic roles of fungi in exacerbating autoimmune symptoms.
The study employed intraperitoneal injection of pristane—a commonly used method to induce lupus-like symptoms in mice. Following the initial treatment, the mice were injected three times a week with C. albicans to observe the combined effects on inflammation and microbiome alterations.
Interestingly, while systemic inflammation indicated by serum cytokines was not significantly altered across the groups, the localized impact on lung inflammation and hemorrhage was profound. The introduction of Candida significantly heightened the local inflammatory response, as demonstrated by elevated cytokine levels specific to lung tissue.
Additionally, the presence of Candida altered the lung microbiome, most notably decreasing proteobacterial abundance and changing alpha and beta diversity metrics compared with the phosphate-buffered solution (PBS) control. This indicates that Candida can influence bacterial populations in the respiratory tract, demonstrating the intricate balance of lung microbiota.
"Due to the presence of Candida in the lower respiratory tract, either with or without lung diseases, Candida administration was tested in vivo," wrote the authors of the article, emphasizing the relevance of understanding microbial interactions in respiratory conditions.
In vitro experiments revealed that when neutrophils were stimulated with a combination of lipopolysaccharides from bacteria along with beta-glucan from Candida, there was a marked increase in pro-inflammatory responses. This suggests a synergistic effect whereby the body’s immune response is significantly magnified in the presence of both fungi and bacterial components.
This research presents critical insights into how the presence of fungal organisms like Candida can exacerbate autoimmune conditions and lung-related pathologies in lupus patients. It highlights the necessity for continued research into how alterations in lung microbiota through fungal colonization might lead to more severe disease manifestations.
Further studies examining the impacts of Candida colonization in human patients’ sputum could aid in understanding the relationship between lung infections and the severity of autoimmune disorders like lupus. Moreover, potential therapeutic strategies targeting the microbial environment in the lungs may help manage or mitigate the severe consequences of lupus-related lung infections.
In conclusion, this study effectively demonstrates how the presence of Candida in the lung exacerbates the severity of lung hemorrhage in a model of lupus-induced DAH. The implications for clinical management of lupus patients include careful consideration of lung health and microbiota balance in the context of immunosuppressive therapies.