In recent years, the therapeutic potential of the psychedelic compound psilocybin, found in certain mushrooms, has garnered significant interest in the realm of psychiatry. Researchers have elucidated the biosynthesis pathway of psilocybin, focusing on how specific enzymes contribute to its production, which could revolutionize treatment options for mental health disorders.
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) has emerged as a prospective candidate for alleviating conditions such as depression and anxiety due to its rapid action and low toxicity profile. The growing prevalence of mental health disorders, often exacerbated by the global pandemic, drives the urgency to develop effective treatments. However, traditional extraction methods of psilocybin from mushrooms are economically unviable for large-scale drug production. Therefore, an alternative route through biosynthesis using engineering microorganisms has been proposed.
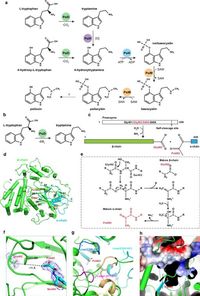
Researchers at institutions studying the enzymes involved in psilocybin synthesis have focused on four key enzymes: PsiD, PsiH, PsiK, and PsiM. In their latest study, they solved the crystal structures of these enzymes to better understand their functions and the biosynthetic pathway overall.
The enzyme PsiD, which catalyzes the conversion of L-tryptophan to tryptamine, showed a unique self-cleavage mechanism, allowing it to function efficiently as a decarboxylase. The involvement of various amino acids within PsiD supports its catalytic activity, and mutations in specific residues resulted in altered enzyme efficiency, hinting at valuable insights for bioengineering improvements.
Researchers explained, "Our study demonstrated that deleting the N-terminal region of PsiD significantly increased its catalytic activity, suggesting a self-inhibition mechanism." This discovery could lead to enhancing psilocybin biosynthesis through genetic engineering, making the production of this promising compound more economical.
Furthermore, the enzyme PsiK’s role in phosphorylating 4-hydroxytryptamine into norbaeocystin was elaborated in this study. The structures revealed crucial insights into the microscopy of any potential inhibitors that may halt the biosynthesis process, which is vital for creating an efficient production pipeline of psilocybin.
The team also explored the antidepressant properties of the biosynthetic intermediates of psilocybin. Female mice, subjected to sub-chronic stress, exhibited improvements in depression-like behaviors after administration of norbaeocystin, supporting the idea of using these intermediates as therapeutic agents. Analysis of behavioral tests indicated that norbaeocystin alleviated symptoms of depression just as effectively as psilocybin itself, without inducing anxiety, a common side effect associated with several traditional antidepressants.
As researchers conclude, "The therapeutic potential of biosynthetic intermediates like norbaeocystin is becoming increasingly clear, providing alternative avenues for treating mental health conditions effectively.” This work sets the stage for future studies to refine psilocybin production and fully realize its potential in clinical settings.
Furthermore, these findings provide a structural basis for bioengineering enhanced production of psilocybin, presenting a unique opportunity to address the vast unmet need for effective treatments for mental health disorders.