As global energy demands rise alongside environmental concerns, researchers are making significant strides in developing sustainable alternatives to fossil fuels. A recent study has achieved a groundbreaking advance in photoelectrochemical (PEC) hydrogen production, demonstrating a record-breaking hydrogen output from crystalline silicon, which has opened new avenues for eco-friendly energy solutions.
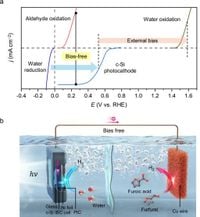
The research, led by a team of scientists, focuses on surpassing the U.S. Department of Energy's (DOE) target for hydrogen production rate of 0.36 mmol h−1 cm−2. By utilizing crystalline silicon—known for its high photocurrent density and stability—the team replaced the conventional water oxidation process with a novel low-potential furfural oxidation reaction. This innovation not only allows for bias-free hydrogen production but also enables the simultaneous generation of hydrogen at both the anode and cathode sides.
In a remarkable achievement, the study has produced hydrogen at a rate of 1.40 mmol h−1 cm−2, exceeding the DOE target by more than fourfold. This breakthrough underscores the potential of PEC technology as a viable means to produce clean fuels from abundant solar energy while addressing pressing environmental issues.
The urgency for sustainable energy solutions has never been greater, as fossil fuel consumption continues to impact the planet's climate negatively. Hydrogen production through achievable solar energy methods represents a promising step towards a cleaner energy future. This study demonstrates that advancements in PEC technology could facilitate a transition away from conventional fossil fuel-based hydrogen production, which currently accounts for more than 90% of hydrogen output globally.
The focus on crystalline silicon (c-Si) as a photoelectrode material is critical, given its theoretical photocurrent density of 43.37 mA cm−2, derived from its small bandgap of 1.1 eV. Despite its high potential, the intrinsic photovoltage of c-Si, approximately 0.6 V, falls short of the necessary voltage for traditional water splitting reactions, which require over 1.6 V.
This study effectively circumvents the photovoltage limitations by employing furfural oxidation instead of water oxidation. The process produces hydrogen gas and valuable furoic acid—a compound with significant industrial uses—while avoiding the less valuable byproducts typically produced through water oxidation. This strategic shift not only drives a higher hydrogen production efficiency but also leverages the high photocurrent of c-Si.
The innovative configuration of the photoelectrode, specifically the p-n junction-based c-Si photocathode utilizing interdigitated back-contact (IBC) structures, enhances energy conversion efficiency. The study indicates that the cooling effect produced by the electrolyte mitigates temperature-induced losses in photovoltage, allowing c-Si photocathodes to function reliably under optimal conditions over extended periods.
Significantly, the research has verified that the integration of a copper (Cu) wire anode in the PEC system effectively supports the low-potential furfural oxidation reaction. By continuously supplying a 50 mM furfural solution, the dual hydrogen production reached a photocurrent density of approximately 40 mA cm−2 while demonstrating high Faradaic efficiency, effectively producing hydrogen without an additional bias.
The comparison of this dual H2 production approach with conventional water-splitting methods highlights the limitations faced by these traditional processes—specifically insufficient photocurrent densities and reliance on extra voltage. The new PEC system outperforms modularized c-Si photocathodes utilized for traditional water splitting without bias, achieving unprecedented efficiencies.
Moreover, the potential for scalable production of furfural from lignocellulosic biomass augments the economic feasibility of this technology long-term. The shift towards renewable resources like furfural paves the way for more sustainable hydrogen production operations that can compete with conventional fossil fuel sources.
The implications of this research are profound, as it lays the groundwork for a sustainable technology capable of producing clean hydrogen on a large scale. By incorporating abundant resources such as lignocellulosic biomass and refining techniques for using other aldehydes, the proposed PEC system could also address broader production challenges for future energy innovations.
This groundbreaking study reveals not only the possibilities of achieving bias-free hydrogen production but also the potential to redefine our approach to renewable energy sources. As substantial momentum builds around PEC technology and further research progresses in this field, the vision of a greener, hydrogen-based economy becomes increasingly tangible.
In conclusion, the advancements made in PEC hydrogen production through innovative applications of crystalline silicon alongside effective use of biomass-derived feedstocks represent a significant leap forward in the quest for sustainable energy solutions. The future of hydrogen production is bright, and with continued innovation, we can hope to meet the global energy demands while safeguarding our planet.