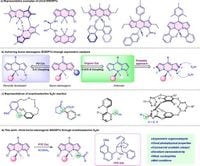
Researchers have unveiled a novel method for synthesizing chiral boron-stereogenic materials that could revolutionize bioimaging techniques. The study, published in 2025, leverages an enantioselective postfunctionalization process for boron dipyrromethene dyes (BODIPYs), expanding their potential applications across various scientific fields.
Boron-containing compounds, particularly BODIPYs, have garnered significant attention in the realms of chemistry, biology, and materials science due to their distinct optoelectronic properties. However, synthesizing chiral BODIPYs has historically posed challenges, primarily due to the limited strategies available for their construction. This new research introduces a phase-transfer-catalyst (PTC) enabled method that allows for the efficient creation of chiral BODIPYs featuring enhanced luminescence and biocompatibility.
The researchers showcased a practical approach by employing an enantioselective C–N coupling reaction, effectively accessing boron-stereogenic amido and amino BODIPYs. "We have developed a practical and universal strategy to access boron-stereogenic amido/amino BODIPYs through phase-transfer-catalyst-enabled enantioselective SNAr reaction," wrote the authors of the article. The method illustrates not only the effectiveness of using various nitrogen nucleophiles but also the potential for significant applications in circularly polarized luminescence (CPL) technology, which includes uses in optical displays and bioprobes.
The results highlighted that the new BODIPYs synthesized demonstrated promising circular dichroism (CD) and CPL activities, yielding products with high specificity and excellent biocompatibility. In particular, emissions were strongly observed in cell applications, with strong green and red luminescence noted in the cytoplasm of treated HeLa cells. These findings indicate a noteworthy advancement in the development of imaging agents that are both effective and environmentally safe.
Traditionally, constructing chiral BODIPYs involved methods that could be cumbersome or limited by specific approaches, like resolution by HPLC or asymmetric synthesis with chiral substrates. Recent advancements in palladium-catalyzed and carbene insertion methods highlighted the feasibility of complex reactions; however, integration remained scarce. With this new phase-transfer-catalysis method, the researchers have provided a pathway that not only increases the efficiency and yield but also places minimal reliance on toxic catalysts which could impede biocompatibility.
The optimization process involved meticulous experimentation. Initially, reaction designs targeting α,α-dichloro BODIPY and benzamide resulted in moderate yielded products. With adjustments in solvents and catalysts, remarkable enantioselectivity values were reached; in one of the key experiments, a specific PTC catalyst led to a 96% enantioselectivity, showcasing that carefully chosen catalysts can dramatically enhance reactions.
Further, the researchers assessed a range of nucleophiles and different BODIPY structures to explore the method's versatility. Amides bearing both electron-donating and withdrawing groups proceeded smoothly, producing various products with impressive yields and enantioselective ratios. "The promising results inspired us to further investigate amines as the nucleophiles," noted the authors of the article, indicating the potential for ongoing research and application in this area.
Through their exploration of BODIPYs, the authors have opened avenues not only for future studies on enhancing fluorescence properties but also for practical applications in medical diagnostics and imaging. The capability to effectively track lipid droplets in live cells underscores the sophistication of this work.
In conclusion, this groundbreaking research exemplifies how innovative methodologies, like the PTC-enablement for enantioselective reactions, can lead to significant advancements in material technologies. By developing safer, more efficient means of synthesizing chiral compounds, this study lays the groundwork for future explorations in the vital field of bioimaging technologies, offering hope for new developments in both scientific and medical applications.