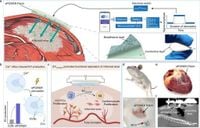
A novel bioelectronic interface technology has emerged that promises a significant breakthrough in treating myocardial infarction (MI), a serious condition characterized by a blockage of blood supply to the heart. Known as the electroactive patch for wirelessly and controllable extracellular vesicle generation (ePOWER), this innovative approach produces bioactive extracellular vesicles (EVs) directly at the site of injury, enhancing cardiac repair and regeneration. Traditional MI treatments often fall short due to their limited efficacy and adverse effects, highlighting the urgent need for more effective solutions.
Myocardial infarction poses a major health challenge globally, contributing to ongoing debates and research focused on improving treatment protocols. Historically, therapeutic approaches like thrombolytic agents and antiplatelet drugs have been the cornerstone in managing MI. However, these strategies carry risks of complications, including excessive bleeding. Furthermore, they fail to adequately address the underlying issue—the heart’s limited ability to regenerate tissue after an injury.
Recent research has turned to cell therapy as a viable alternative, particularly focusing on the potential of extracellular vesicles. EVs, natural nanosized lipid bilayers loaded with bioactive molecules, derived from cells, can facilitate intercellular communication and hold promise for enhancing tissue repair. However, approaches involving the exogenous delivery of EVs face challenges regarding scalability and precise targeting during administration.
The ePOWER technology addresses these deficiencies by utilizing a bioadhesive cardiac patch embedded with electrosensitive macrophages. When electrically stimulated, these macrophages produce a significantly greater number of bioactive EVs—approximately 2400% more per cell than under standard conditions— and deliver them directly to the site of injury. This ensures targeted treatment, bypassing systemic complications associated with traditional therapies.
The researchers conducted experiments using rat models of myocardial infarction to demonstrate ePOWER's therapeutic potential. Initial results showed that when surgically implanted, this patch not only produced EVs but also actively contributed to reducing inflammation and improving overall cardiac function. As the authors noted, "Our results suggest the successful activation of the ePOWER system to produce EVs in vivo under wireless control." This innovative approach allows for a high degree of programmability and control over the biological activity that the patch can induce.
Furthermore, these EVs exhibited anti-inflammatory properties that not only reduced inflammation at the site of MI but also facilitated a shift in macrophages from a pro-inflammatory M1 phenotype to a reparative M2 phenotype, crucial for effective healing.
Each aspect of the technology represents a significant advancement in the field; its ability to produce high concentrations of therapeutic EVs on demand could revolutionize how clinicians approach MI treatment. Importantly, the implications of this research extend beyond cardiac applications. The ePOWER system’s versatility may allow it to be adapted to treat other diseases, such as diabetes, spinal cord injuries, and even cancer treatments in the future.
The systematic analysis of the adhesive properties of the ePOWER patch also demonstrated that it maintains a secure fit on the heart, essential for generating effective treatment and reducing the risk of complications from device implantation. The studies used detailed imaging techniques to confirm that the patch remained intact and functional throughout the treatment period.
As MI continues to be a leading cause of mortality worldwide, strategies that promote cardiac regeneration are vital. The integration of ePOWER technology into clinical practice could greatly enhance the efficacy of MI treatment, potentially leading to better patient outcomes and paving the way for new therapeutic avenues in regenerative medicine.
In summary, the introduction of the ePOWER system represents a transformative step in healing the heart after myocardial infarction, combining cutting-edge bioelectronic interfaces with the therapeutic properties of extracellular vesicles. With further research and clinical validation, ePOWER may soon become a vital tool in cardiac rehabilitation and beyond.