The Agência Nacional de Vigilância Sanitária (Anvisa) has taken the precautionary step of banning the sale of Colgate Clean Mint toothpaste after receiving numerous reports of adverse reactions from consumers. This decision, published in the Diário Oficial on March 27, 2025, prohibits the commercialization of the product throughout Brazil for a period of 90 days, although Colgate has the option to appeal the ruling.
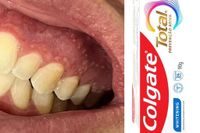
According to Anvisa, the agency received eight notifications involving 13 cases of adverse events related to the use of Colgate's toothpaste between January 1 and March 19, 2025. The reported symptoms include swelling of the tonsils, lips, and oral mucosa, as well as burning sensations, numbness in the lips and mouth, dry mouth, irritated gums, and redness.
The affected product, Clean Mint, is a new version of the popular Colgate Total 12 toothpaste, which underwent a formula change before its launch in July 2024. The new formulation replaced sodium fluoride with stannous fluoride, a change that the company claims is safe and effective. Colgate asserts that the new formula is the result of over a decade of research and extensive consumer testing, including trials conducted in Brazil.
Despite Colgate's assurances regarding the safety of the new formula, some consumers have reported severe reactions. Alina Dourado, a 30-year-old user, experienced symptoms shortly after switching to the new product. "I noticed a sore on the underside of my tongue, and as days passed, more sores appeared around it. The area under my tongue became so swollen that it affected my speech," she recounted. After consulting health professionals and undergoing various tests, she ultimately decided to stop using the toothpaste, which led to an immediate improvement in her symptoms.
Dental professionals have weighed in on the situation as well. Aline Laignier, a pediatric dentist and orthodontist, noted that allergic reactions to toothpaste can manifest in various ways, including burning sensations and sores. "It's important to highlight that other components in toothpaste can also cause allergies, not just stannous fluoride. Some patients may react to flavorings or detergents in the product," she explained.
The Conselho Regional de Odontologia de São Paulo (CROSP) has also commented on the issue, stating that adverse reactions to toothpaste typically cease once the product is discontinued. They advise consumers who experience any sensitivity to stop using the product immediately and seek alternatives.
Colgate has acknowledged that while the vast majority of users will not experience issues, a small minority may have sensitivities to certain ingredients. In a statement, the company reiterated that stannous fluoride is a widely used and safe ingredient in toothpastes globally. They recommend that consumers who experience adverse reactions discontinue use to alleviate symptoms.
Additionally, the company has set up a customer service line for affected consumers to report their experiences and seek assistance. The number provided is 0800 703 7722.
Consumer feedback has been largely negative since the formula change, with many users expressing their concerns on various platforms. Notably, influencer Luiza Guimarães shared her struggles with mouth sores that only subsided after she stopped using the toothpaste. "You would never think a toothpaste would cause allergies, especially one that you've used all your life," she lamented in a social media post.
Another user, 25-year-old Pedro Maciel, reported a similar experience, stating he developed numerous sores in his mouth after using the new formula. "I had over 50 sores at one point, which lasted nearly a month. I consulted four doctors, and no one could provide a diagnosis. I was given medication to treat the symptoms but ultimately had to stop using the toothpaste," he said.
As the situation develops, Colgate is under scrutiny not only from consumers but also from regulatory bodies. Anvisa's decision reflects a growing concern over consumer safety and the potential health risks associated with personal care products.
The agency will continue to monitor the situation, and the outcome of Colgate's potential appeal remains to be seen. For now, the interdiction serves as a warning to both consumers and manufacturers regarding the importance of ingredient safety and transparency in personal care products.
In the meantime, consumers are advised to remain vigilant and report any adverse reactions they may experience while using dental products. The importance of thorough testing and consumer feedback cannot be overstated, as they play critical roles in ensuring public health and safety.