Colorectal cancer (CRC) has emerged as a pressing global health challenge, standing as the third most common malignancy and the second leading cause of cancer-related death worldwide. Recent advances have highlighted the crucial role of the immune system, particularly tertiary lymphoid structures (TLS), in influencing CRC progression and patient outcomes. TLS maturation, characterized by the presence of germinal centers, has been linked to better prognosis and immunotherapy responses. However, accurately assessing TLS has proven difficult due to inconsistencies in histological definitions and the limitations of traditional staining methods.
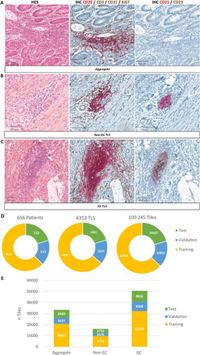
A recent study conducted by researchers at CHU Brest, France, sought to overcome these challenges by using an AI-based approach for automatic classification of TLS in colorectal adenocarcinoma patients. The study involved a comprehensive cohort of 656 patients whose tissue samples were examined through dual immunohistochemistry staining for CD21 and CD23, markers important for classifying TLS maturation stages. The researchers employed advanced machine learning algorithms, including ResNet50 and a Vision Transformer model, to analyze whole-slide images (WSI) of the tissue samples.
As TLS play a pivotal role in regulating immune responses within the tumor microenvironment, understanding their maturity could provide critical insights into treatment efficacy. The study's algorithm, termed TLS-PAT, achieved impressive accuracy levels, with an accuracy score of 0.845 and a kappa value of 0.761, indicating robustness in its classification capabilities. These findings represent a significant advancement in the standardization of TLS assessment, which is essential for informing clinical decisions in CRC treatment.
The research design included a sophisticated methodology involving five-fold cross-validation to ensure the reliability of the results. The dataset was meticulously annotated by trained pathologists, who classified the TLS according to established criteria, resulting in a well-structured and balanced collection of TLS from the patients. The model evaluations indicated that the Vision Transformer architecture, particularly when pretrained on ImageNet and utilizing a Max Confidence aggregation method, yielded superior classifying performance compared to the ResNet50 model.
A pivotal aspect of this study was the evaluation of interobserver agreement among pathologists using Cohen's kappa statistics, which demonstrated substantial consistency in the classification of TLS. This robust inter-rater reliability reinforces the potential of automated classification systems, as they could lead to more objective and reproducible assessments than traditional manual evaluations.
Additionally, the study revealed critical insights into the performance of different aggregation methods used in the classification process. While various techniques were assessed, the Max Confidence method proved to be the optimal choice, prioritizing the class with the highest confidence score, which markedly improved accuracy. The model displayed remarkable capability in differentiating between various stages of TLS maturation, specifically achieving up to 98.93% accuracy for germinal center-like TLS.
In a head-to-head comparison of TLS-PAT with human pathologists, the AI tool demonstrated comparable accuracy but significantly outperformed pathologists in classification speed. For instance, while a trained pathologist could achieve a classification accuracy of 90% in approximately 10 minutes, TLS-PAT accomplished the same task in just 15 seconds. This efficiency demonstrates the tool's practical implications for clinical workflows, offering not only quicker assessments but also consistent results.
The study results indicate a tangible impact on CRC patient care by streamlining the TLS classification process, which could lead to increased accuracy in prognostic determinations and more personalized treatment approaches. TLS-PAT offers pathologists a supportive tool that integrates seamlessly with existing clinical practices while contributing to enhanced objectivity in tissue analysis.
Despite these promising results, the authors acknowledge that further validation is necessary to ensure these findings are generalizable across diverse patient populations and cancer types. Testing across different cancers and integrating external datasets will be crucial for refining the approach and enhancing its applicability.
In conclusion, this innovative AI-driven approach represents a significant leap forward in the automated analysis of TLS in colorectal cancer. By standardizing the assessment process and improving objectivity, the TLS-PAT tool might play an essential role in routine clinical practice, ultimately benefitting patients through more tailored cancer therapies.