In a striking discovery, researchers have unveiled how acute immune activation—common during critical illnesses—can inadvertently lead to a dangerous proliferation of harmful gut bacteria. This process, largely due to a shift in the delicate balance of gut microbiota, is particularly concerning in critically ill patients, who often exhibit elevated levels of lipopolysaccharide (LPS) following endotoxemia.
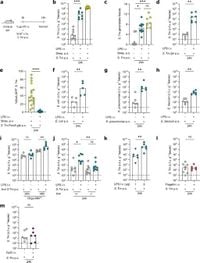
The study, conducted in mouse models, provides new insights into how systemic immune responses to LPS can promote a 100 to 10,000-fold increase in opportunistic pathogens like Klebsiella pneumoniae, Escherichia coli, Enterococcus faecium, and Salmonella Typhimurium within just one day. Notably, these developments occur without the overt signs of gastrointestinal inflammation typically associated with bacterial infections.
“A transient inflammatory pulse induced by LPS leads to the release of oxygen species into the gut lumen,” the authors of the article stated. This shift allows facultative anaerobic pathogens—bacteria that can thrive in both oxygen-rich and oxygen-poor environments—to flourish at the expense of beneficial microbiota.
This phenomenon highlights a paradox: while the immune system 'wakes up' in response to an insult, it simultaneously compromises the very microbial defenses that reside within our intestines. The findings underscore the importance of maintaining microbial homeostasis in patients experiencing critical illnesses.
Previous research has established the gut microbiota's role in deterring opportunistic pathogens via competitive inhibition; when it is disrupted—often by external factors such as antibiotics or acute inflammation—pathogens can seize the opportunity to multiply unchecked.
This study meticulously tracked the outcomes of intravenous LPS injections in mice, noting that systemic immune activation significantly impeded the fermentation processes crucial to gut health. The immune response spurred by LPS increased gut-luminal levels of reactive oxygen species (ROS), creating an environment that facilitated the growth of pathogenic bacteria.
“Our findings establish a direct link between acute immune activation and microbiota perturbations,” the researchers noted. It was found that while the levels of opportunistic pathogens soared, there was a concerning decline in beneficial microbial fermentation, which normally helps produce short-chain fatty acids essential for gut health.
Interestingly, this bloom of pathogens was shown to occur independently of classic virulence mechanisms typically associated with gut infections. In the experiments, pathogen blooms emerged even when virulence factors like the Type III secretion system were absent, indicating that pathogens can exploit changes in the intestinal milieu brought about by immune activation.
Critically, the research suggests that such opportunistic infections are a significant risk factor for critically ill patients, especially those with compromised microbiota due to prolonged hospital stays or antibiotic treatments.
Moreover, beyond simply increasing the prevalence of pathogens, systemic immune activation also seems to foster their systemic dissemination. The findings observed a rise in pathogen loads within mesenteric lymph nodes and the spleen, regionally indicating the risks associated with these gut blooms.
This interplay between immune response and microbial dynamics poses pressing questions for healthcare. For instance, how can clinicians sculpt the gut environment to maintain microbial homeostasis in patients vulnerable to infections?
As for potential interventions, there are emerging thoughts on employing non-pathogenic competitor strains to regulate the gut microbiota and mitigate risks associated with opportunistic pathogens.
Finally, while these findings significantly deepen our understanding of gut microbiota responses during critical illness, further research is necessary to elucidate the precise mechanisms at play, including the various oxygen species involved and their sources during immune activation.
In conclusion, this study sheds light on a critical component of infectious disease risk in hospitals and offers a nuanced view on how transient immune changes can create environments ripe for pathogen overgrowth. The results suggest new possible therapeutic strategies to protect vulnerable patients in acute care settings from the dire consequences of limb through opportunistic infections.