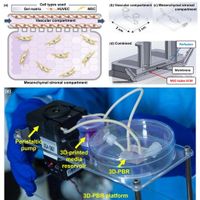
The field of regenerative medicine has gained a significant boon with the introduction of a novel fabrication method for creating a 3D-printed perfusion bioreactor (3D-PBR), which facilitates the in situ growth and differentiation of human bone marrow-derived mesenchymal stem cells (MSCs) while enabling effective coculture with vascular cells. This innovative approach, outlined by researchers at Los Alamos National Laboratory, combines essential characteristics like reproducibility, biocompatibility, and ease of customization, creating a microenvironment that accurately recapitulates human tissue physiology.
Much of the progress in microphysiological systems (MPS) has focused on organ-on-a-chip designs where the intricacies of human tissues are modeled in vitro. The traditional methods have often fallen short in mimicking the complex microenvironment found in the human body, particularly the bone marrow, an organ essential for regulating blood cell development and bone health. The 3D-PBR addresses these challenges through its unique design that allows MSCs to co-culture with human vascular cells, facilitating the differentiation of these stem cells into specific lineages, such as adipocytes (fat cells) and osteoblasts (bone-forming cells).
The 3D-PBR features a biocompatible resin-based polymer that is 3D-printed with precision. Following the initial printing process, a permeable membrane is introduced to create two fluid compartments—the vascular section and the mesenchymal stromal (MS) compartment. This design aids in enhancing the communication necessary for cellular development and function. The vascular channel measures 11.8 mm × 4.8 mm × 0.8 mm, while the MS compartment is 15.8 mm × 4.8 mm × 0.8 mm, allowing for efficient media flow, crucial for nutrient exchange and effective differentiation.
As described in the research, the MSCs are encapsulated in a collagen-fibrin gel within the MS compartment. The physical cues that the 3D-PBR provides were found to be pivotal in guiding the differentiation of MSCs. In laboratory tests, the engineering of the bioreactor proved effective, as evidenced by immunohistochemistry images that indicated a more physiologically relevant state compared to conventional flat static culture systems.
During the experimental phase, researchers assessed cell viability using Acridine Orange/Propidium Iodide (AO/PI) staining, establishing a mean viability rate of 92% for MSCs and 91% for human umbilical vein endothelial cells (HUVECs). These high viability rates highlight the success of the 3D-PBR in fostering a suitable growth environment.
After a 21-day period of differentiation, both the MSCs and HUVECs demonstrated mature cellular phenotypes indicative of successful adipogenic and osteogenic development. Interestingly, the study noted that mechanical forces exerted by fluid flow within the 3D-PBR play a critical role in enhancing osteogenesis—a key insight for future bioprinting endeavors in tissue engineering.
"The dynamic 3D tissue microenvironment provided by the 3D-PBR may promote a more physiologically relevant differentiation process for MSCs," remarked the authors of the article. This underscores the 3D-PBR's ability to simulate lived experiences more accurately, opening innovative avenues for understanding stem cell behavior and interaction in the human body.
The implications of this advanced bioreactor are manifold, promising enhancements in drug testing strategies, regenerative medicine, and possibly superior outcomes in tissue-engineered grafts. By integrating vascular and mesenchymal compartments, the researchers have created a versatile platform for revealing new insights into how different cell types interact and function, fundamentally contributing to our understanding of hematopoiesis and bone tissue regeneration.
As the researchers move forward, they aim to explore deeper functionalities of the 3D-PBR, refining its designs and applications. Their ongoing work hopes to reveal the mechanobiological signals that regulate stem cell fate, potentially leading to transformative applications in treating bone-related disorders and other diseases. The novel fabrication method confirms that 3D printing technologies can significantly enhance the capacity of in vitro systems, propelling research in regenerative medicine towards new frontiers.