Recent research has unveiled intriguing insights about the effects of ionizing radiation, particularly focusing on the potential benefits of low-dose exposure. The study, which investigated the role of priming doses of radiation, revealed significant protective effects on cellular response to subsequent higher doses. Researchers discovered evidence indicating how pretreatment with 0.5 Gy of ionizing radiation can reduce DNA damage and cell death among lung cells.
The study, published on March 18, 2025, involved experiments conducted primarily at the Atomic Energy Authority, Cairo, Egypt, and aimed to clarify existing uncertainties surrounding low-dose ionizing radiation (LDIR). It is well-known within the scientific community how ionizing radiation can produce biological effects through interactions at the cellular level. At higher doses, radiation’s link to biological consequences is clearer; but at lower doses, the cellular response is more complex and under investigation. Low-dose radiation, defined as doses of less than 200 mGy, may lead to non-targeted effects such as the adaptive response.
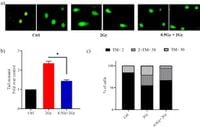
Adaptive response refers to the phenomenon whereby cells demonstrate increased resistance to subsequent higher doses of radiation after being pre-exposed to low doses. The importance of this phenomenon was highlighted when researchers found statistically significant differences between lung fibroblast cells treated solely with high doses of 2 Gy and those treated with both the 0.5 Gy priming dose and 2 Gy. The latter group showed reduced DNA damage following irradiation and maintained higher cell viability, demonstrating the protective qualities of the priming dose.
Applying various scientific techniques, the team examined cellular behavior post-radiation exposure. Using comet assays—a method to quantify DNA damage—the researchers discovered the impact of the 0.5 Gy dose was protective, lowering the total extent of damage observed compared to treatments with the 2 Gy dose alone. Cells pre-treated exhibited reduced levels of apoptosis, which can be defined as programmed cell death, compared to those exposed to the challenge dose alone.
Through rigorous assessments, the study also yielded insights on molecular pathways triggered by the low-dose exposure. It was established, for example, cycling analysis indicated cells pre-exposed to 0.5 Gy transitioned from the G1 phase of the cell lifecycle more effectively than those subjected only to the higher 2 Gy dose. Specifically, only 12% of cells treated solely with the higher dose showed typical mitotic catastrophes, or severe disruptions during cell division, compared to just 7% of cells exposed to both doses.
“Pretreatment with 0.5 Gy protected lung fibroblasts from DNA damage caused by a subsequent 2.0 Gy dose administered 24 hours later,” wrote the authors of the article, summarizing one of their key findings. This highlights the potential application of low doses of radiation, possibly leading to new clinical strategies for radiation therapies.
Overall, the study presents meaningful contributions to the discussions surrounding radiation exposure and its mechanisms. Researchers acknowledge the complex nature of assessing risk when dealing with ionizing radiation, especially concerning public health and environmental safety. They aim to deepen the dialogue around how these findings can be coherently applied within clinical realms, exploring possibilities for enhancing patient safety and treatment efficacy.
Looking to the future, more investigations are necessary to delineate the underlying mechanisms through which 0.5 Gy radiation communicates protective cellular responses. The advancements here could revolutionize how we approach both cancer treatment protocols and protective measures for households or industries using ionizing radiation.